O Level Chemistry Practicals
O Level Chemistry Practical
This practical class is designed to help students excel in the practical component in both IP and Chemistry O Level Practical examinations. At Seb Academy, the practical papers set for the students involved the investment of many hours of experimental design, and testing for students’ safety, and careful calibration of quantities for students to obtain accurate results on their own. All the experiments were designed with the intention of helping students conquer the practical examination and secure a strong grade for the final examinations for the GCE O Level, hence would involve some question spotting for the examinations.
Guided Chemistry Practical Experiment
At Seb Academy, our goal is not to only provide a comprehensive laboratory, but to equip students with proficient practical knowledge. More importantly, it is to help students appreciate Chemistry better through very meticulously set practical assessments. Through the lab sessions, we also want to ensure that students can also prepare themselves better for the practical assessments in school.
What sets our practical preparatory classes apart is we guide students in our introduction classes on tips in titration such as pipetting, collection of gases and QA to save time and adopt valuable practical tips to ace the Chemistry practical examinations.

Meticulously Planned
Designing original experiments is extremely time-consuming. We have poured many hours into planning and executing the experiments to cultivate critical thinking while helping students excel in the exams better. Each practical exam assessment at Seb Academy is designed to help students tackle the O Level / IP Chemistry practical examinations.
The response from students and academic results have proven that the design and intent of the practicals to help students master the experiment component of the Chemistry examinations has been extremely well.
Curated O Level Syllabus and carefully planned Experiments
The lab classes are created with the aim to inculcate an appreciation of the various forms of qualitative, and quantitative analysis to help students understand the theory component of the subject better. such Different analysis includes thermogravimetric and kinetic analysis applied with rigorous quantitative analysis (mole calculations).
Special notes are given to our students when we teach and explain our intent when stepping into Chemistry topics such as Chapter 18 Rate of Reactions. Running a Chemistry practical is one thing. However, it is troubleshooting and data handling, in addition to managing the Chemistry Practical that would truly help students set their grade apart for the O Level / IP Practical assessment.
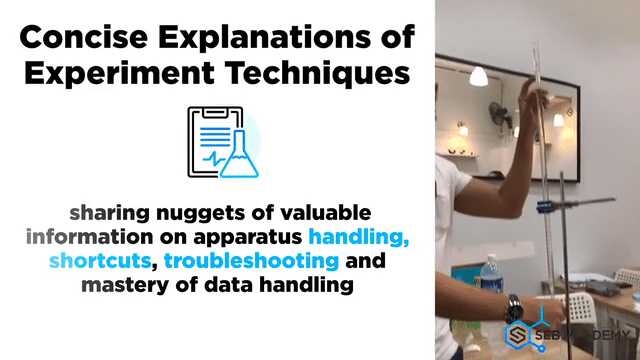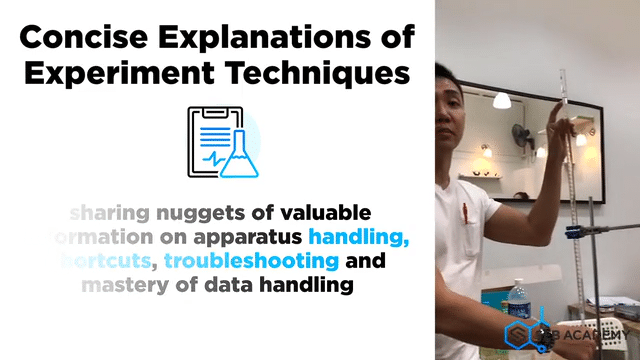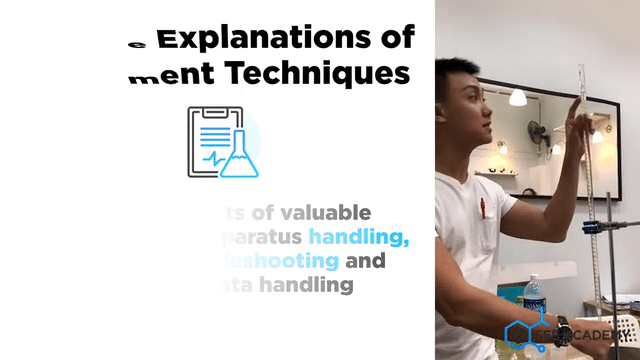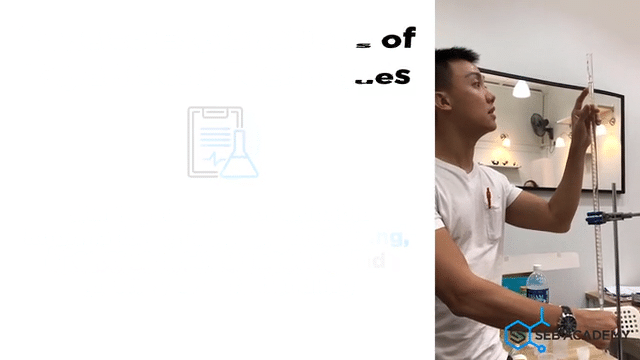
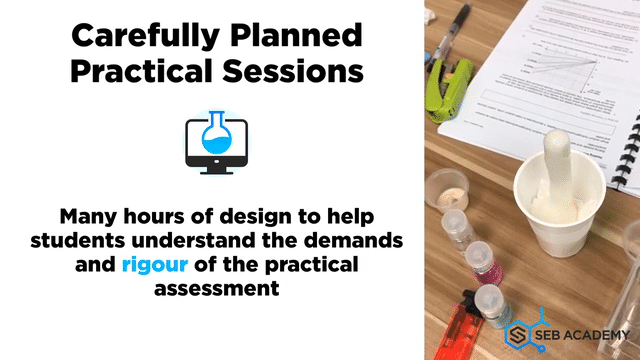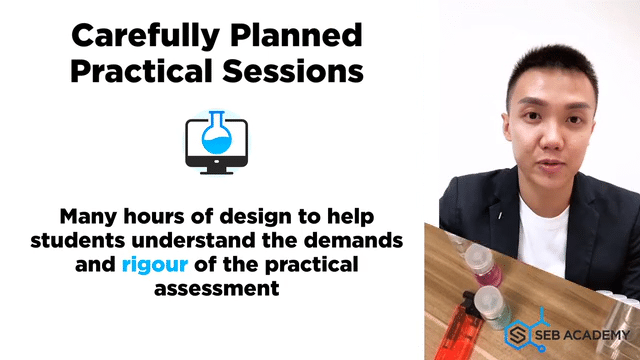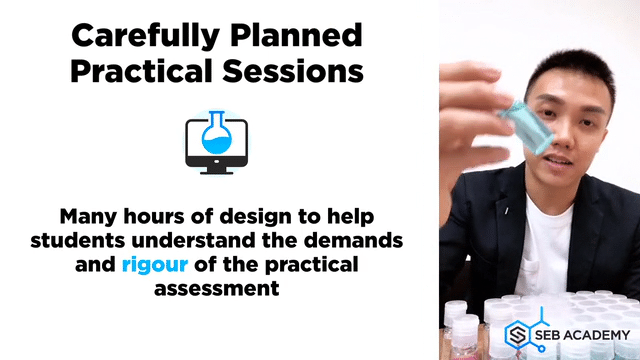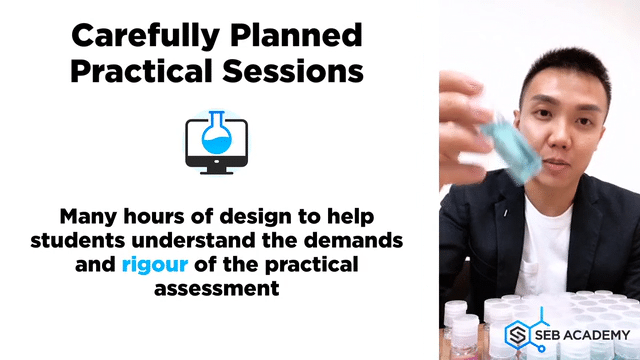
Carefully planned Chemistry Planned Assessment
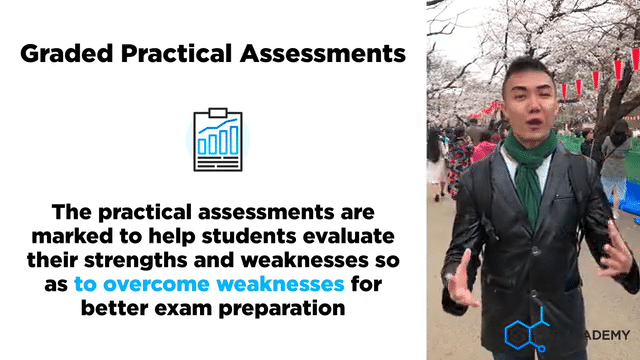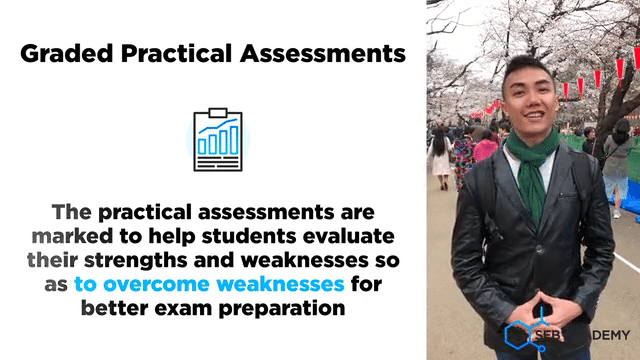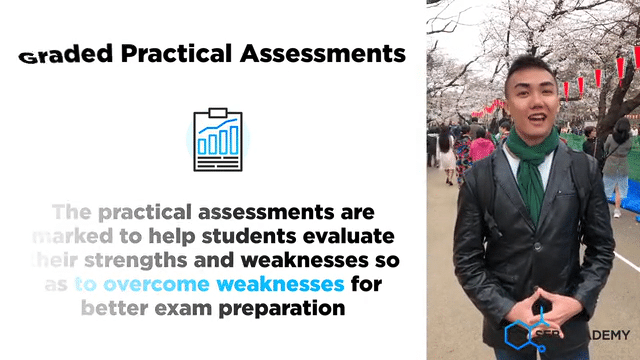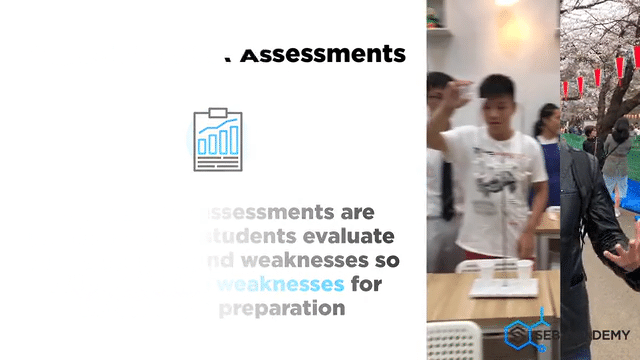
A lot of careful deliberation effort has been put into designing, curating, setting up, overseeing, grades and assessing students in the mastery of Chemistry lab sessions. The practical aims to equip students with the necessary skills to handle lab apparatus safely and demonstrate a fair understanding of analysing chemicals. We grade our students in their Chemistry Practical assessments to help students understand their strengths and weaknesses better
Graded Chemistry Practical Assessments & Evaluations
Post mock practical examinations at Seb Academy, we set up Zoom classes to run through the concepts and common practical fraughts for the O Level Chemistry / IP Practical from their sessions and individual feedback for students practical skills on how to conduct the experiments.

All chemicals are sourced from safe and approved materials
All our chemicals obtained are NEA-approved and are safe for lab use. The Chemistry experiments have been performed by our teachers before the practical to assess the safety and efficacy of the practical to ensure students would be able to perform the practical examinations in our lab safely, on time, and with efficacy to deliver the critical thinking component required for the O level Chemistry / IP Practical examinations.
Exam Coaching
The entire idea for O level Chemistry lab lessons and practical is to support students in their academic pursuits and excellence through cultivating hands-on observations deductions and intuitive conclusions of chemical experiments. Through more hands-on practical sessions, students would be better equipped to handle the O level Chemistry Practical Examinations.

Chemistry O Level Practical Paper Breakdown
The Chemistry Paper for Practical is also known as Paper 3 of the O level Practical Paper. This paper consists of a variable number of compulsory practical questions. This usually involves acid-base titration or redox titration. One, or more, of the questions may incorporate assessment of Planning (P) and require candidates to apply and integrate knowledge and understanding from different sections of the syllabus. The assessment of PDO and ACE may include questions on data analysis that do not require practical equipment and apparatus.
| Paper | Type of Paper | Duration | Marks Weighting |
|---|---|---|---|
| 1 | Multiple Choice (40 questions) | 1H | 40 |
| 2 | Structured and Free Response Section A (50 marks; 7-8 questions) Section B (30 marks; 3questions) | 1 h 45 min | 80 |
| 3 | Practical (40 marks; 3-4 questions) | 1 hr 50 min | 40 |
Types of Questions that can be tested in the Practical Paper:
- Acid-base titration using methyl orange as indicator for colour changes.
*Students are required to note colour changes and titration results in suitable tables and perform satisfactory quantitative analysis.
2. QA: Test of Gases: Carbon dioxide, Hydrogen, Oxygen, Ammonia gas
3. QA: Test of Gases: Test of cations and anions
4. Thermometric analysis involving temperature change in a styrofoam cup.
5. Speeds of reaction involving usage of a stopwatch
6. various other forms of practical such as paper chromatography.
In the practical exam, candidates will be assessed in the following skill areas:
(a) Planning (P)
Candidates should be able to:
• identify key variables for a given question/problem such as testing the effect of catalyst on the speeds of reactions.
• outline an experimental procedure to investigate the question/problem, i.e. usage of methyl orange to perform titration
• describe how the data should be used in order to reach a conclusion, e.g. testing hydrogen gas should a metal react with acid
• identify the risks of the experiment and state precautions that should be taken to keep risks to a minimum, especially in titrations involving redox or acids, or loss of heat energy in energy changes questions.
(b) Manipulation, measurement and observation (MMO)
Candidates should be able to:
• set up apparatus correctly by following written instructions or diagrams, in preparation for titration or QA
• use common laboratory apparatus and techniques to collect data and make observations, such as the use of silver nitrate acidified or barium nitrate acidified to test for anions.
• describe and explain how apparatus and techniques are used correctly, to test for gases when effervescence is observed.
• make and record accurate observations with good details and measurements to an appropriate
degree of precision,
• make appropriate decisions about measurements or observations

(c) Presentation of data and observations (PDO)
Candidates should be able to:
• present all information in an appropriate form and tables, displaying numbers and other tabulated resources in neat and legible form
• manipulate measurements effectively for analysis, in preparation for drawing logical conclusions from tests and data to deduce a logical answer for the question.
• present all quantitative data to an appropriate number of decimal places/significant figures. Take note students, 3sf still applies!
(d) Analysis, conclusions and evaluation (ACE)
Candidates should be able to:
• analyse and interpret data or observations appropriately in relation to the task
• draw conclusion(s) from the interpretation of experimental data or observations and underlying principles
• make predictions based on their data and conclusions to satisfactory quality for examiners
• identify significant sources of errors and explain how they affect the results especially in titrations
• state and explain how significant errors may be overcome or reduced, as appropriate, including how experimental procedures may be improved
One, or more, of the questions may incorporate some assessment of skill area P, set in the context of the syllabus content, requiring candidates to apply and integrate knowledge and understanding from different sections of the syllabus. It may also require the treatment of given experimental data in drawing relevant conclusion and analysis of the proposed plan.
The Planning Question:
The planning question is compulsory, and is worth 5-6 marks and is one common pitfall for students taking the practical exams. Questions in the practical planning can include acid titration, salt preparation, experimental chemistry, or testing variables for kinetic or energy changes of reactions. It is not an impossibility to rule out ratio analysis in mole concept and stoichiometry as such students should add mole practice to their repertoire or preparation for the practical exam.
The GCE O Level Requirements by SEAB:
Candidates should be able to use appropriate apparatus/equipment to record a range of measurements such as mass, length, time, volume and temperature. In addition, candidates are expected to have been exposed to a range of experimental techniques in the following areas:
1. Titration
e.g. acid-base titration (with suitable indicators such as methyl orange, screened methyl orange, and thymolphthalein). Other types of titrations may also be required, and where appropriate, sufficient working details will be given.
2. Speeds of reaction
that may involve measuring of quantities, e.g. temperature, volume, length, mass or time measurements
3. Separating Techniques
Experiments involving separation techniques such as simple paper chromatography, filtration and distillation
4. Salt preparation
5. Gas collection
6. Qualitative inorganic analysis involving an element, a compound or a mixture, including displacement reactions and tests for oxidising and reducing agents. Candidates should be familiar with the reactions of cations, reactions of anions and tests for gases as detailed in the Notes for Qualitative Analysis.
Candidates would not be required to carry out tests involving Pb2+ ions or sulfur dioxide gas.
Reactions involving ions not included in the Notes for Qualitative Analysis may be tested: in such cases, candidates will not be expected to identify the ions but only to draw conclusions of a general nature.
Candidates should not attempt tests, other than those specified, on substances, except when it is
appropriate to test for a gas.
7. Qualitative organic analysis
Requiring knowledge of simple organic reactions as outlined in Section VI,
e.g. test-tube reactions indicating the presence of unsaturation (C=C) may be set, but this would be for
the testing of observation skills and drawing general conclusions only.
Interested to find out more? Come join us at Seb Academy!
We want to see you excel! For more enquiries about the practical introductory classes, or mock practical examinations at Seb Academy, do feel free to contact us for more information.
| Date | Time | Topic | Teacher | Venue |
|---|---|---|---|---|
| 6/10 Wed | 9 -10.00am | Chem QA session 1 (Combined Sciences) | Mr Sebastian Lim | Bishan |
| 6/10 Wed | 10.00 - 11.00am | Chem QA session 2 (Combined Sciences) | Mr Sebastian Lim | Bishan |
| 7/10 Thu | 9 -10.50am | Energy and Rates + Planning (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 7/10 Thu | 11-12.50pm | Energy and Rates + Planning (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 11/10 Mon | 9 -10.30am | Titration (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 11/10 Mon | 10.30 -12pm | QA (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 11/10 Mon | 1-2.30pm | Titration (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 11/10 Mon | 2.30 -4pm | QA (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 12/10 Tue | 9 -10.30am | Energy + Rates (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 12/10 Tue | 10.30 -12pm | Exp Planning (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 12/10 Tue | 1-2.30pm | Energy + Rates (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 12/10 Tue | 2.30 -4pm | Exp Planning (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 13/10 Wed | 9 -10.50am | Titration + QA Session 1 (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 13/10 Wed | 11-12.50pm | Titration + QA Session 1 (Pure Chemistry) | Mr Sebastian Lim | Bishan |
| 13/10 Wed | 2-3.50pm | Titration + QA Session 1 (Pure Chemistry) | Mr Sebastian Lim | Bishan |
This form is currently closed for submissions.
FAQ
Have any questions? Reach us or click here to see our FAQ