Sec 3 Homework Review
-
C1 Particle Theory Homework
-
C3 Separating Techniques Homework
-
C4 Atomic Structure Homework1Topic
-
C6 Chem Bonding Homework4Topics
-
C7 Chemical Equations1Topic
-
C9 Mole Concept Homework3Topics
-
C11 Acids, Bases and Salts Homework3Topics
-
C12 Salt Prep3Topics
-
C13 Redox Homework2Topics
-
C14 Metals Homework3Topics
-
C16 Periodic Trends1Topic
-
C25 QA3Topics
-
Sec 3 Term 3 Homework3Topics
-
EOY Sec 3 Homework (4)5Topics
C3 Separating Techniques Homework 1 Quiz
Quiz Summary
0 of 25 Questions completed
Questions:
Information
You have already completed this quiz. You cannot start it again.
Quiz is loading…
You must sign in or sign up to take this quiz.
You must first complete the following:
Results
Quiz complete. Results are being recorded.
Results
0 of 25 Questions answered correctly
Your Time:
Time has elapsed.
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
-
It’s ok. Let’s try it again. The road to success starts when you simply try!

-
You are getting it! Keep on trying! Text me any questions you have. We can do this together. Take a screenshot of this and select the whatsapp icon on the top left hand corner, and whatsapp me. – Mr Lim

-
Almost there! Keep on at it! Text me any questions you have. Every step you take, you are nearing towards your goal. Take a screenshot of any questions you have, select the whatsapp icon on the top left hand corner, and whatsapp me. – Mr Lim

-
 A2! Keep on at it! Text me any questions you have. Take a screenshot of this, select the whatsapp icon on the top left hand corner, and whatsapp me. – Mr Lim
A2! Keep on at it! Text me any questions you have. Take a screenshot of this, select the whatsapp icon on the top left hand corner, and whatsapp me. – Mr Lim -
A1! Good job! If you have any questions, do text me. Take a screenshot of the question, select the whatsapp icon on the top left hand corner, and whatsapp me. – Mr Lim

-
Distinction! Bravo. – Mr Lim

| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
Would you like to add your score to the leaderboard?
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 251. Question
A mixture of sulfur and iron filings needs to be separated. The solubilities of sulfur and iron filings in water and carbon disulfide are shown in the table below.

What are the possible methods of separating the sulfur and iron filings?
 CorrectIncorrect
CorrectIncorrect -
Question 2 of 252. Question
(2018/SNGS/FE/1) What is the most appropriate sequence of method(s) that can be carried out to separate pure, dry copper(II) oxide from a mixture of zinc oxide and copper(II) oxide?
CorrectIncorrect -
Question 3 of 253. Question
(2018/S3HC/Set A) Which of the following mixture can be separated into its components by adding water, stirring filtering?
CorrectIncorrect -
Question 4 of 254. Question
(2012/S3RP/MYCT) Which of the following processes is most suitable for separating a solid mixture of zinc nitrate and calcium carbonate?
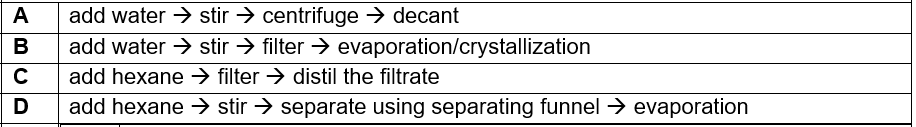 CorrectIncorrect
CorrectIncorrect -
Question 5 of 255. Question
A bottle of solid copper(II) oxide has been contaminated with some solid sodium chloride. How can sodium chloride be removed from the copper(II) oxide?
CorrectIncorrect -
Question 6 of 256. Question
(2018/NYGH/S3/IP/FE/P1/4) Lead (II) sulfate is soluble in hot water but almost insoluble in cold water. Which of the following methods is most suitable for obtaining a pure sample of lead (II) sulfate crystals from a flask containing a hot aqueous solution of lead (II) sulfate and sodium sulfate?
CorrectIncorrect -
Question 7 of 257. Question
(2018/RP/S3/IP/FE/P1/4) The reaction scheme shows how hydrated copper (II) sulfate, CuSO4 5H2O, changes when heated.
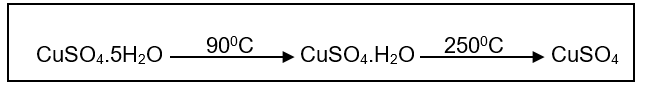
A little water was accidentally spilled into a dish containing hydrated copper (II) sulfate.
What could be done to remove the water, leaving pure, dry CuSO4 5H2O?
CorrectIncorrect -
Question 8 of 258. Question
Gore-tex fabric is a type of waterproof and breathable material often used in outdoor sportswear. It is able to block out rainwater and wind. While allowing water vapour from body perspiration to escape. Its structure consists of a few layers of porous membranes, each with pores of different sizes. Which of the following explains how the fabric can be water-resistant and yet breathable?
CorrectIncorrect -
Question 9 of 259. Question
(2012/S3RP/MYCT) Which of the following processes is most suitable for separating a solid mixture of zinc nitrate and calcium carbonate?
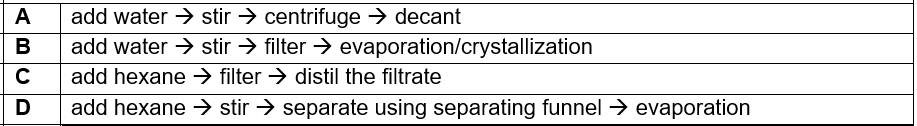 CorrectIncorrect
CorrectIncorrect -
Question 10 of 2510. Question
Which one of the following is suitable for extracting the water from a solution containing sugar?
CorrectIncorrect -
Question 11 of 2511. Question
(2013/VS/S3/IP/CA1/5) A student tried to separate seawater by distillation using the apparatus shown below.
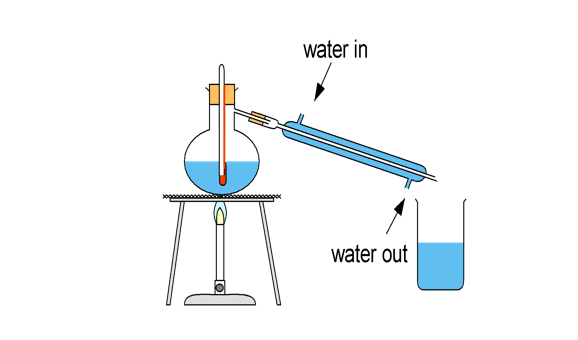
What are the errors made by the student?
 CorrectIncorrect
CorrectIncorrect -
Question 12 of 2512. Question
(2018/CGSS/FE/1) Which mixtures of substances can be separated using the apparatus below?
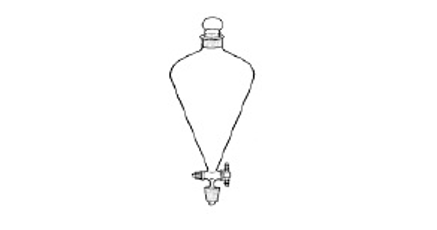 CorrectIncorrect
CorrectIncorrect -
Question 13 of 2513. Question
Which mixture can be best separated by fractional distillation?
CorrectIncorrect -
Question 14 of 2514. Question
(2017/NYGH/S3/IP/FE/P1/5) Which of the following properties provide the basis for separation using fractional distillation?
CorrectIncorrect -
Question 15 of 2515. Question
(2018/NYGH/S3/IP/FE/P1/3) Which of the following mixtures of substances can be separated using a separating funnel?
CorrectIncorrect -
Question 16 of 2516. Question
(2018/NYGH/S3/IP/FE/P1/3) Which of the following mixtures of substances can be separated using a separating funnel?
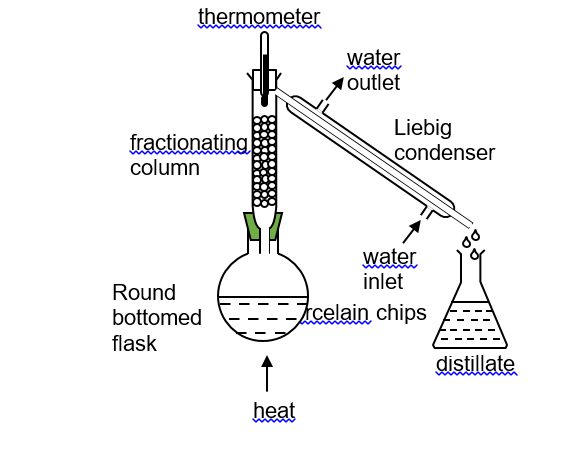
 CorrectIncorrect
CorrectIncorrect -
Question 17 of 2517. Question
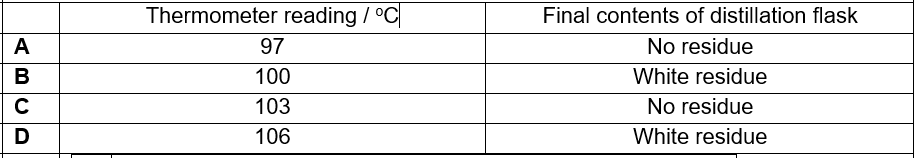
(2018/TKGSS/FE1) A mixture containing equal volumes of two liquids that mix completely but do not react together is placed in the apparatus shown and heated until the thermometer first shows a steady reading. At which position will there be the highest proportion of the liquid with the higher boiling point?
 CorrectIncorrect
CorrectIncorrect -
Question 18 of 2518. Question
(2010/HC/S1) The diagram shows the apparatus being used to distill seawater. At which points will the temperature be 100 oC?
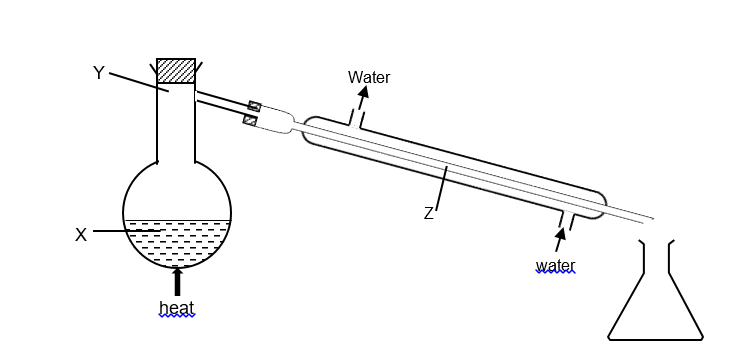 CorrectIncorrect
CorrectIncorrect -
Question 19 of 2519. Question
(2014/S4NYGH/IPFE/2) The apparatus below can be used to extract propanone, C3H6O (boiling point 56°C) from a solution of propanone containing plant dyes.
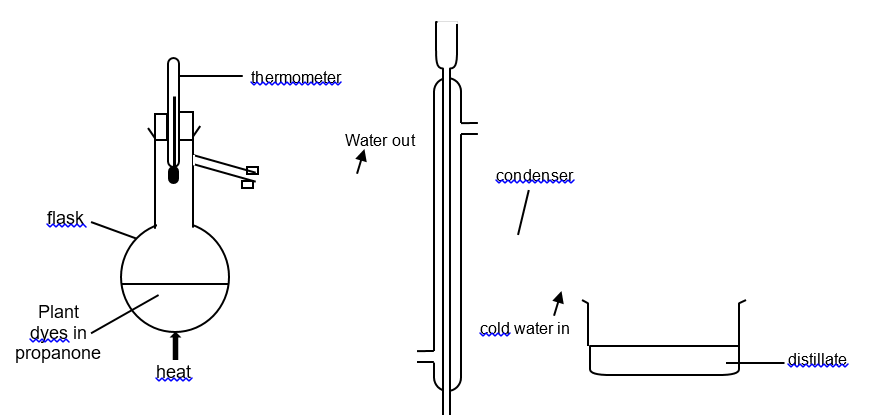
Which of the following statements is true about the plant dyes during the separation process?
CorrectIncorrect -
Question 20 of 2520. Question
(2017/NYGH/S3/IP/FE P1) Which of the following properties provide the basis for separation using fractional distillation?
CorrectIncorrect -
Question 21 of 2521. Question
(2013/VS/S3/IP/SA1/7) The diagram shows the fractional distillation apparatus used to separate alcohol and water, which has a boiling point of 78 °C and 100 °C respectively.
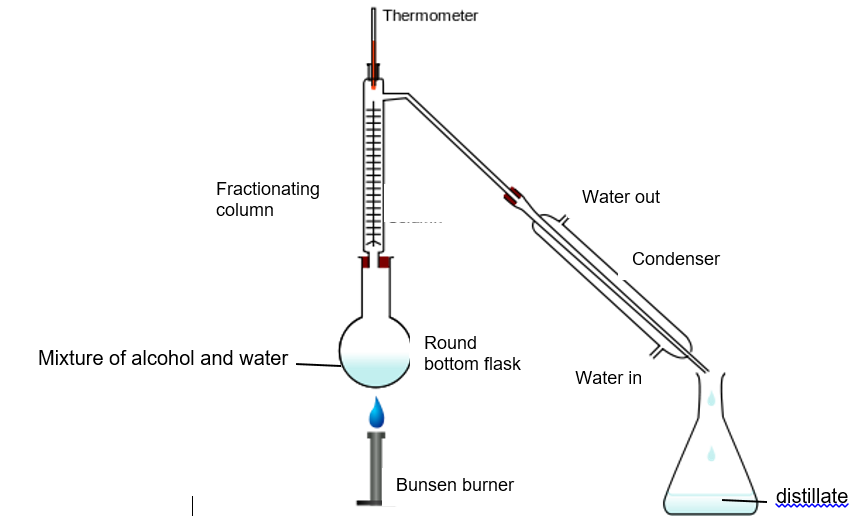
Which of the following graphs best shows how the temperature varied with the volume of distillate collected?
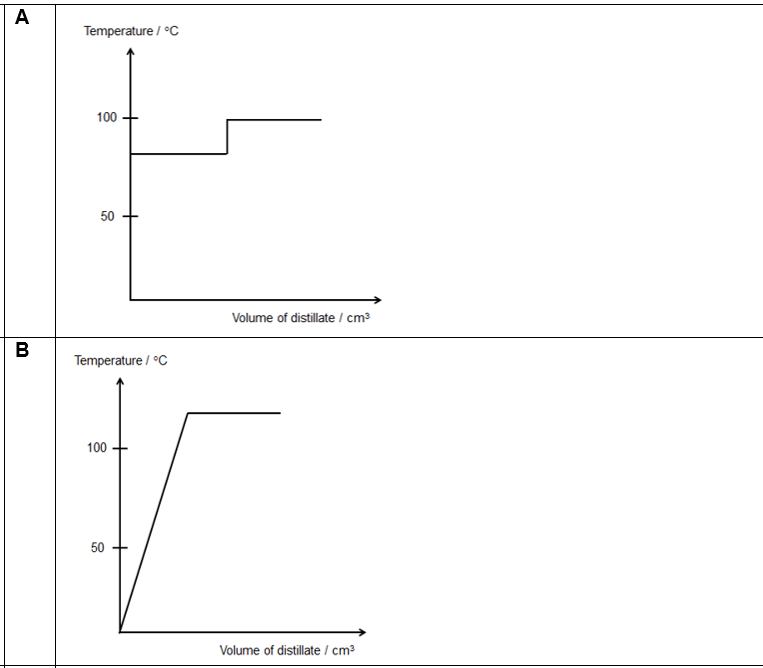
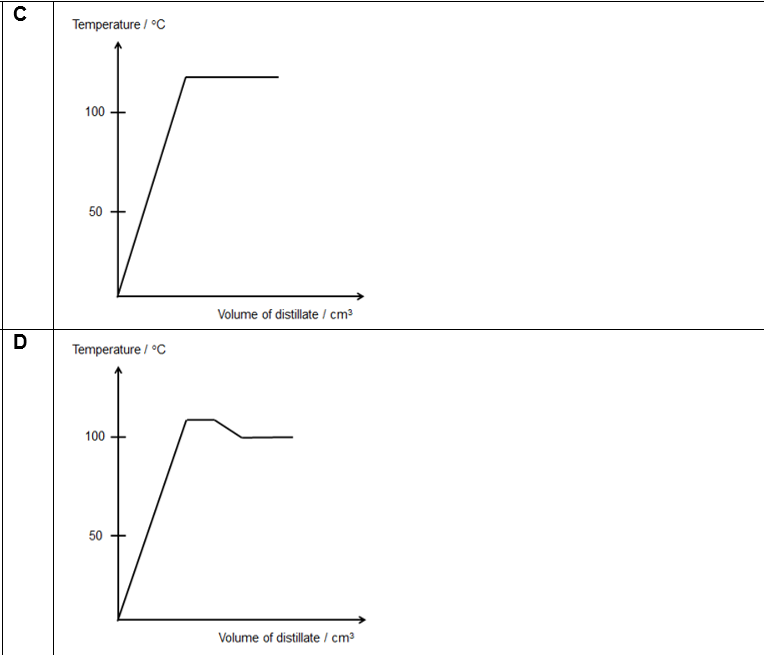 CorrectIncorrect
CorrectIncorrect -
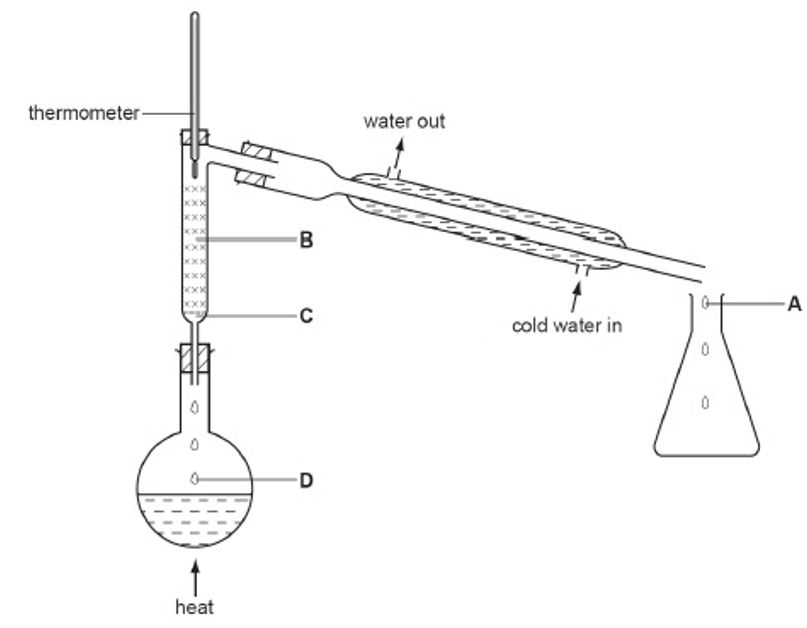
Question 22 of 2522. Question
(2015/RP/S3/IP/FE/P1/5) A mixture of water and ethanol is being separated using the apparatus shown below.
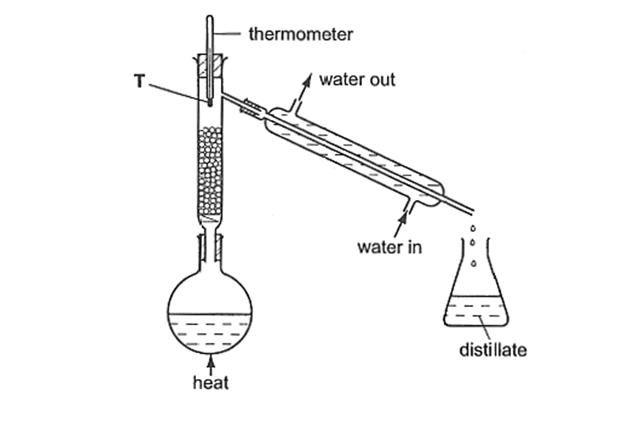
Which statement about the process is false?
CorrectIncorrect -
Question 23 of 2523. Question
(2017/S3RP//MYCT) Toluene and cyclohexane are two common organic solvents and they are miscible in each other. The following graph shows the boiling points of mixtures containing different proportions of toluene and cyclohexane.
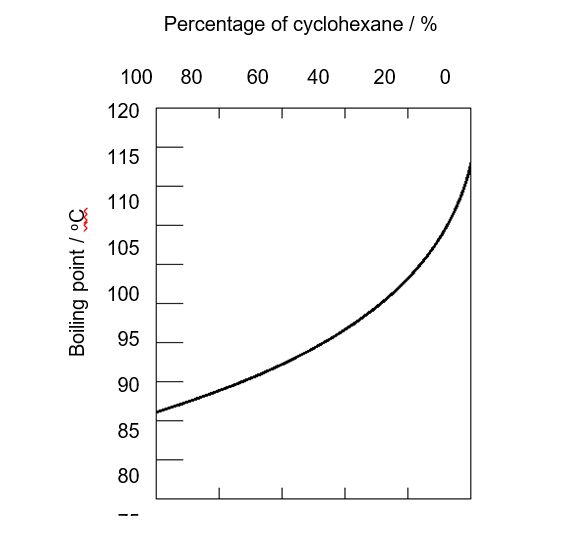
Which of the following statements(s) is/are correct?
1. The mixture can be separated by fractional distillation.
2. The boiling point of toluene is lower than that of cyclohexane.
3. Both toluene and cyclohexane are soluble in water.
CorrectIncorrect -
Question 24 of 2524. Question
The following graph shows the temperature changes when a 1:1 mixture of methanol (melting point – 97 oC, boiling point 65 oC) and propan 2-ol (melting point – 89 oC, boiling point 82 oC) was distilled.
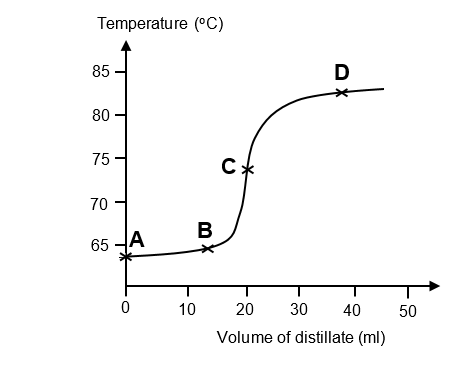
If a 2 ml fraction of the distillate were collected at each of the points A, B, C and D indicated on the graph, which fraction would contain the highest proportion of methanol?
CorrectIncorrect -
Question 25 of 2525. Question
(2012/S3RP//MYCT) Two liquids, X and Y, are placed in a separating funnel. Two layers are formed as shown in the diagram below.
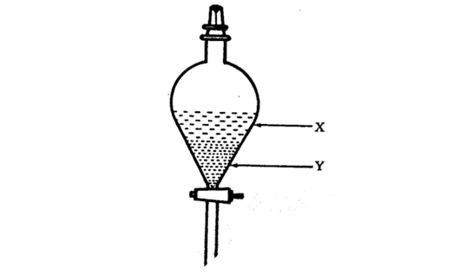
From this, it can be deduced that liquid Y _____________________.
1. has a higher density than X
2. Is water
3. has a lower boiling point than liquid X
CorrectIncorrect
