Titrations Level 1: Basics
Titrations Level 1: Basics
Quiz Summary
0 of 10 Questions completed
Questions:
Information
You have already completed this quiz. You cannot start it again.
Quiz is loading…
You must sign in or sign up to take this quiz.
You must first complete the following:
Results
Quiz complete. Results are being recorded.
Results
0 of 10 Questions answered correctly
Your Time:
Time has elapsed.
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
Would you like to add your score to the leaderboard?
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 101. Question
How many moles of HCl are needed to react with 0.25 moles of Na2CO3?
Na2CO3(aq) + 2HCl2(aq) = 2 NaCl(aq) + CO2(g) + H2O(l)
Equation 5: Neutralization of Sodium Carbonate with HCl.
CorrectIncorrect -
Question 2 of 102. Question
Which of the following is NOT an acid-base indicator?
CorrectIncorrect -
Question 3 of 103. Question
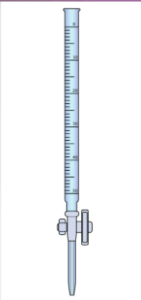
What is the smallest reading we should record to, on a typical burette?
 CorrectIncorrect
CorrectIncorrect -
Question 4 of 104. Question
What is the correct reading on this burette?
 CorrectIncorrect
CorrectIncorrect -
Question 5 of 105. Question
Phenolphthalein is added as an indicator. What is wrong with this?
CorrectIncorrect -
Question 6 of 106. Question
What is the average titre of the experimental volumes 24.20cm3, 24.50cm3, 24.55cm3 and 24.60cm3
CorrectIncorrect -
Question 7 of 107. Question
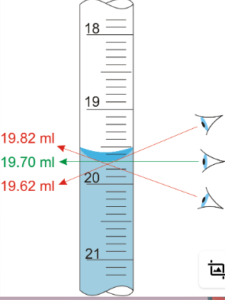
What is this type of error called?
 CorrectIncorrect
CorrectIncorrect -
Question 8 of 108. Question
What is the correct formula for calculating an unknown concentration? n = moles, v = volume in dm3
CorrectIncorrect -
Question 9 of 109. Question
The point at which the indicator changes colour in a titration is called the…
CorrectIncorrect -
Question 10 of 1010. Question
How many moles are there in 25cm3 of a 0.100 moldm-3 solution of KCl?
CorrectIncorrect
